Background:CLL is the most common form of leukemia in pts aged ≥ 19 y and accounts for 38% of all new leukemia cases. The CLL treatment (tx) paradigm changed after the introduction of oral targeted therapies (Bruton tyrosine kinase inhibitors [BTKi] and B-cell lymphoma 2 inhibitors). However, relapses after tx are common, and these txs come with a high cost. Limited data exist evaluating the lifetime cost of treating pts with CLL with current therapies. The objective of this study was to estimate the total lifetime cost of treating pts with CLL in the US from the health system perspective.
Methods: A semi-Markov model with a lifetime horizon was developed to estimate the per-pt cost of treating CLL. The model consisted of 6 distinct health states, including tx by line of therapy (LOT; first line [1L] through fifth line and later [5L+]) and death. Pts entered the model in the 1L. CLL txs selected for inclusion were those most commonly used according to market share data and recommended by National Comprehensive Cancer Network guidelines. Tx categories included ibrutinib ± anti-CD20 monoclonal antibody (mAb), acalabrutinib ± anti-CD20 mAb, venetoclax-based regimens, chemoimmunotherapy, and phosphatidylinositol 3-kinase inhibitor-based regimens. Transition probabilities and time spent on each LOT were calculated based on data abstracted from digitized time to next tx (TTNT) and OS Kaplan-Meier curves from real-world studies. Tx-specific data were applied for 1L and second-line (2L) txs, and data pooled across txs for each LOT were applied for third line (3L) through 5L+. TTNT and OS data from the literature were extracted for the duration of available follow-up. Best-fitting parametric survival models were used for reasonable extrapolation of TTNT data beyond the limited follow-up. OS beyond the available follow-up was estimated based on survival data from US life tables adjusted for each tx via a calculated tx-specific HR. Costs associated with drug acquisition and administration of CLL txs, inpatient stays, outpatient visits, emergency department (ED) visits, Richter's transformation (RT), end-of-life care, and other health care resource utilization (HCRU) were included in the model. AE management costs were assumed to be captured within inpatient costs and therefore not included separately in the model. Inputs were derived from published literature and publicly available sources. At the point of entry into the model, the study cohort was assumed to be 70 y old and included 64% males, based on median age and sex distribution reported in the literature (Mato et al. Clin Lymphoma Myeloma Leuk 2023). All costs were converted to a health system perspective, discounted 3% per year, and adjusted to 2023 US dollars (USD). Model outcomes included per-pt total expected lifetime cost and total expected lifespan. A scenario analysis was conducted to calculate per-pt-per-year (PPPY) cost of each LOT. A 1-way sensitivity analysis was conducted to identify key drivers of the total lifetime cost.
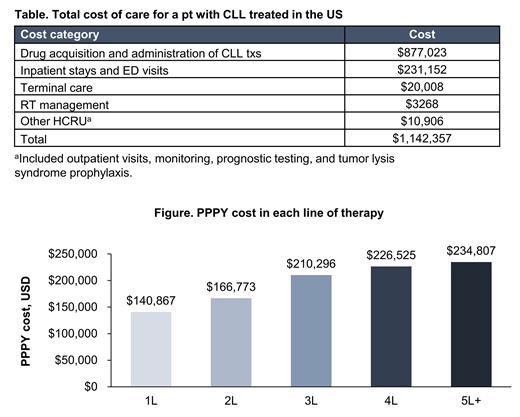
Results: The total expected lifetime cost of treating a pt with CLL in the US was estimated to be $1,142,357. The estimated total time spent in the model was 8.03 y. Drug costs were the largest driver of the total lifetime cost (77%), followed by inpatient and ED costs (20%; Table). The majority of time was spent in 1L and 2L (6 and 1.68 y, respectively). The probability of receiving 2L, 3L, fourth-line (4L), and 5L+ therapy was estimated to be 44%, 16%, 6%, and 4%, respectively. In the scenario analysis, the annual cost in each LOT ranged from $140,867 to $234,807 PPPY, with costs increasing with each subsequent LOT (Figure). The 1-way sensitivity analysis showed that lifetime costs were mainly driven by drug acquisition costs, especially BTKi such as ibrutinib and acalabrutinib, as well as the cost of venetoclax. Other key drivers of the total expected lifetime cost included hospitalization costs and pt starting age.
Conclusions:Study results showed that the expected lifetime cost of treating a pt with CLL in the US is high. Drugs (especially oral targeted txs) were the top drivers of lifetime total cost. As expected, scenario analysis showed that the economic burden increased with each LOT. Given the high lifetime economic burden of this disease, there is a need for potentially more effective 1-time-only tx options.
Disclosures
Awan:Pharmacyclics LLC, an AbbVie Company.: Other: Contracted Research; Janssen, Gilead, Kite pharmaceuticals, Karyopharm, MEI Pharma, Verastem, Incyte, Johnson and Johnson, Merck, Epizyme, Loxo Oncology, Adaptive Biotechnologies, Genmab: Other: Consulting Agreements; AstraZeneca Pharmaceuticals LP: Other: Advisory Committee; AbbVie Inc, ADC Therapeutics, AstraZeneca Pharmaceuticals LP, BeiGene Ltd, Bristol-Myers Squibb Company, Cardinal Health, Caribou Biosciences Inc, Celgene Corporation, Cellectar Biosciences Inc, DAVA Oncology, Epizyme Inc, Genentech, a member of the Roche: Other: Consulting Agreements. Vidisheva:Bristol Myers Squibb: Consultancy. Saeedian:Bristol Myers Squibb: Consultancy; BluePath Solutions: Current Employment. Kurt:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Tiwana:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Priyadarshini:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal